Understanding Late Leakage in Softgel Capsules: Causes and Prevention
Late-stage leakage is one of the most frustrating phenomena in softgel development. In any given project, softgel capsules pass initial quality control and survive months of stability testing, but can then begin leaking. Understanding why some capsules fail immediately while others develop leaks months later is essential for robust formulation development and predicting long-term product stability.
This article examines the mechanisms behind delayed leakage and provides practical strategies for identifying at-risk formulations before they reach the market.
The Time of Leakage: Immediate vs. Delayed Failures
Not all leakers are created equal. Immediate leakage, which occurs within hours or days of manufacturing, typically signals seam defects.
Late-stage leakage is fundamentally different, however. The capsules appear perfect initially, passing all standard quality tests. The failure mechanism is time-dependent, often requiring months to manifest. The root cause is usually a slow evolution of the capsule system itself: changes in water and plasticizer content of the shell, gradual chemical interactions, or progressive physical incompatibilities that develop as the formulation moves toward equilibrium.
The distinction matters because it determines your development strategy. Immediate leakers can be identified and corrected quickly. Late-stage failures require a deeper understanding of formulation dynamics.
Want to master advanced leak prevention strategies? Our online course covers formulation approaches and analytical techniques for predicting long-term stability, based on real-world manufacturing experience.
Migration: The Slow-Motion Culprit
Migration of plasticizer and water represents the most common mechanism behind delayed leakage. At the microscopic level, softgel systems are never truly static. Components continuously redistribute between fill and shell, seeking thermodynamic equilibrium. This process, while often slow, fundamentally alters the properties of both phases over time.
When shell plasticizers and water migrate into the hydrophilic fill, the shell loses flexibility and becomes increasingly brittle. Thermal cycling during storage or shipping then provides sufficient stress to crack the weakened shell, often at the seam or in high-stress areas.
The rate of migration depends on concentration gradients between fill and shell, molecular weight of migrating species, temperature, and specific interactions between components. Importantly, migration can continue for months after encapsulation, meaning your capsules at six months may have different properties than at six weeks.
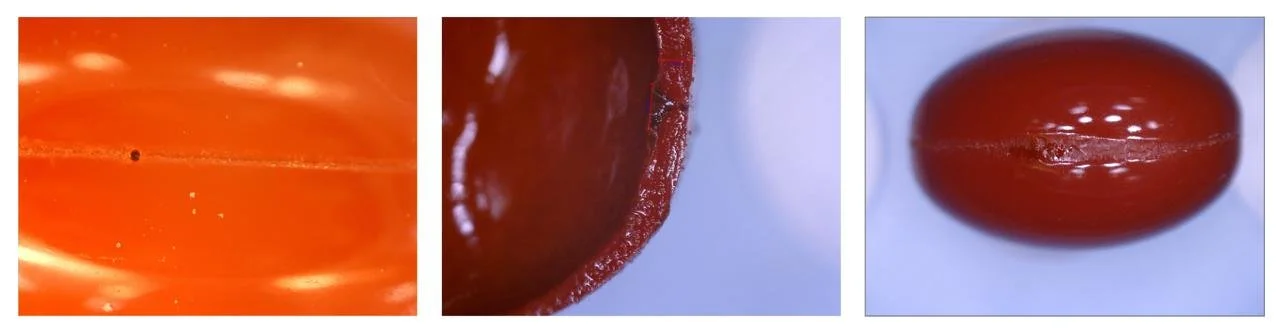
Pinhole leakers
Storage Conditions and Environmental Stress
Environmental conditions during storage and distribution profoundly influence when and whether capsules will leak. In fact, temperature fluctuations deserve particular attention because they affect softgel systems through multiple mechanisms simultaneously.
Higher temperatures accelerate every time-dependent process in your formulation. Migration rates increase exponentially with temperature following Arrhenius kinetics. Shell plasticizer distribution shifts, potentially creating localized regions of excessive softness or brittleness.
Temperature cycling presents an additional challenge. Each cycle creates thermal expansion and contraction, placing mechanical stress on the seal. While a single cycle causes no visible damage, repeated cycling during shipping or in poorly controlled warehouses can progressively weaken seals already compromised by migration effects.
Humidity plays a crucial role. Low humidity accelerates moisture loss, potentially making shells even more brittle. This occurs especially if the capsules are packed into materials with a high water vapor transmission rate (WVTR).
The Accelerated Stability Paradox
Accelerated stability testing creates a fundamental tension in softgel development. We use elevated temperatures to compress months of aging into weeks, assuming that failure modes at 40°C accurately predict what happens at 25°C, just faster. This assumption doesn't always hold for complex softgel systems.
Migration rates do increase predictably with temperature, and this aspect works reasonably well. However, the relationship between shell properties and temperature is not purely linear. Some formulations that remain stable at room temperature become unstable through different mechanisms at elevated temperatures. A shell composition that maintains adequate firmness at 25°C might become excessively soft at 40°C, even without additional migration.
This creates scenarios where accelerated testing either underestimates or overestimates real-world performance. The solution is not to abandon accelerated testing—it remains invaluable for early screening—but to recognize its limitations. Critical formulations require real-time stability data, particularly when pushing boundaries with challenging fills. Accelerated conditions should raise flags for deeper investigation, not serve as the sole basis for shelf-life determinations.
Struggling with stability prediction for challenging formulations? Our online course covers analytical methods and interpretation strategies that help identify problems before they reach commercial scale.
Predicting Late-Stage Leakage: Beyond Standard Testing
Effective prediction of delayed leakage requires moving beyond routine quality control to interrogate the fundamental stability of your formulation.
Early time-point analysis of migration in high-risk formulations can reveal problems long before visible leakage occurs. Analyzing shell and fill composition at multiple time points during manufacturing stability shows whether significant migration is occurring and in which direction. Changes in shell moisture content, plasticizer levels, or fill water activity provide early warning signs of formulations moving toward problematic equilibrium states.
Mechanical testing at multiple time points provides objective data on how shell properties evolve. Simple penetration tests reveal whether shells are becoming progressively softer or more brittle.
Prevention Strategies: Formulation and Process Controls
Preventing late-stage leakage begins with formulation design that accounts for long-term equilibrium states, not just initial compatibility. The goal is to create systems that reach stable equilibria quickly and maintain integrity throughout the product lifecycle.
Plasticizer selection and optimization deserve renewed attention when designing for long-term stability. As discussed in our plasticizers article, migration tendencies vary significantly between different plasticizing agents. For fills containing hydrophilic components, consider plasticizer systems with higher molecular weights or reduced migration potential. The shell should maintain adequate flexibility even if some plasticizer loss occurs, while avoiding excess softness if migration from the fill is likely.
Fill formulation adjustments can eliminate driving forces for problematic migration. Matching the water activity between fill and shell reduces the thermodynamic gradient driving water transfer. Including small amounts of shell plasticizers in the fill formulation—particularly when those components would naturally migrate anyway—can establish equilibrium more quickly and at more favorable compositions.
Our online softgel course is now live!
After years of requests, we've packaged our expertise into comprehensive training courses:
+ Practical formulation knowledge based on 15+ years of experience
+ Solutions to common quality issues like leakage and crosslinking
+ Available on-demand access—learn whenever and wherever you want